Abstract
Background: Duvelisib (DUV), an oral, first-in-class dual inhibitor of PI3K-δ,-γ, is being developed for the treatment of hematologic malignancies, including relapsed/refractory (RR) CLL/SLL. In the Phase 3 DUO study (NCT02004522) DUV monotherapy demonstrated a significant improvement in efficacy compared to ofatumumab (OFA) monotherapy (median PFS [mPFS] 13.3 vs 9.9 mo., p<0.0001; ORR 74% vs 45%, p <0.0001) with a manageable safety profile (Flinn, Blood, in press). Study IPI-145-12 (NCT02049515) is an open-label, optional, crossover extension study where patients (pts) with radiologically confirmed progressive disease (PD) on DUO were given the option to receive the opposite treatment. Here we present data for the 90 pts who crossed over following PD on OFA (pre-crossover) and received DUV on Study IPI-145-12 (post-crossover).
Methods: Eligible pts enrolled within 3 months of PD on the DUO study (excluding Richter's transformation or prolymphocytic leukemia). DUV 25 mg BID was administered until PD, intolerance, death, or study withdrawal. Responses were determined by investigators per IWCLL (Cheson 2011)/IWG criteria (Cheson 2007) with modification for treatment-related lymphocytosis.
Results: As of 15 June 2018, 90 pts crossed over from OFA on the DUO Study to receive DUV monotherapy 25 mg BID on Study IPI-145-12. At the initial study entry (DUO) the median age was 68 yrs (range: 39-89); 63% were male; and 90% Caucasian. Nearly half (49%) of the pts had Rai Stage III/IV or Binet Stage C, and 23% had del(17p) and/or TP53 mutation. The median number of prior anticancer therapies was 3 (range: 2-8), with 60% of pts having received ≥ 3 prior anticancer therapies.
90 of the 101 pts treated with OFA on DUO who had PD crossed over to receive DUV. The median exposure to OFA for these 90 pts was 23 weeks (range: 1-26 weeks), with 61% receiving all 12 prescribed infusions. Post-crossover, the median exposure to DUV was 43 weeks (range: 2-170 weeks).
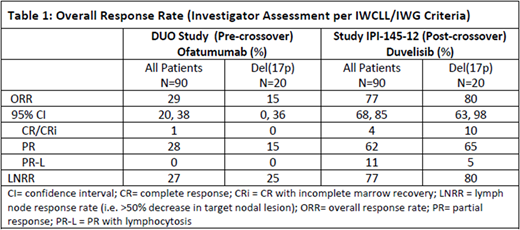
Table 1 shows the overall response rate for the same pt set pre-crossover on OFA and post-crossover on DUV.
The mPFS on DUV post-crossover was 15 months (95% CI: 12, 20) compared to a mPFS of 9 months (95% CI: 9, 11) on OFA pre-crossover. This difference was even more pronounced in pts with del(17p) (n=20), who had a mPFS of 17 months (95% CI: 9, 21) on DUV post-crossover compared to a mPFS of 8 months (95% CI: 5, 11) on OFA pre-crossover. The ORR on DUV post-crossover was 77% compared to 29% on OFA pre-crossover, and in the subset of pts with del(17p) was 80% on DUV vs 15% on OFA. In the subset of pts (n=47) who had no response on OFA pre-crossover (SD/PD), the ORR post-crossover on DUV was 73%. Pts on DUV achieved rapid lymphocytosis (median 1.1 months) with a median time to first response of 2.6 months (i.e., first response assessment).
The most common severe (≥ Gr 3) hematologic AEs on DUV included neutropenia (23%) and thrombocytopenia (4%). The most common severe non-hematologic AEs included diarrhea (21%), pneumonia (12%), colitis (11%), lipase increased (7%), acute renal failure (6%), and bronchitis, rash, and sepsis (4%, each). Nine pts had AEs with a fatal outcome with 1 assessed as related to treatment (PJP). Approximately 70% of pts had a dose modification (interruption and/or reduction) due to an AE, with the most common (>5%) being diarrhea (27%), pneumonia (10%), colitis (8%), neutropenia and lipase increased (7% each). AEs leading to treatment discontinuation (≥ 2 pts) included colitis (n=9; 10%), diarrhea (n=8; 9%), pneumonia (n=2; 2%), and rash (n=2; 2%).
Seventy-three pts (81%) have discontinued DUV on Study IPI-145-12, 39 (43%) due to AEs, 19 (21%) due to PD, 6 (7%) due to death, 5 (6%) due to voluntary withdrawal/investigator decision, and 4 (4%) for other reasons; 17 (19%) pts remain on DUV.
Conclusions: DUV monotherapy achieved robust and durable responses (ORR 77%, mPFS 15 months) in pts with RR CLL/SLL who had PD following OFA monotherapy on the DUO study, including pts with del(17p) (ORR 80%). DUV also showed strong activity (ORR 73%) in pts who had no prior response on OFA. Response on DUV was rapid, with a median time to lymphocytosis of ~ 1 month and a median time to response of ~2 months (first response assessment). The safety profile of DUV monotherapy in this crossover study was manageable and similar to that observed with DUV monotherapy on the DUO study. These data further support the potential clinical benefit of DUV monotherapy in pts with RR CLL/SLL.
Davids:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Research Funding; Surface Oncology: Research Funding; Merck: Consultancy; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding. Hillmen:Alexion Pharmaceuticals, Inc: Consultancy, Honoraria; Pharmacyclics: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Gilead Sciences, Inc.: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Montillo:Roche: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria. Lamanna:TG Therapeutics: Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jannsen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding. Tam:Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Stilgenbauer:Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffmann La-Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmcyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ghia:Acerta: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; Sunesis: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie, Inc: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Le:Verastem Oncology: Employment. Lustgarten:Verastem Oncology: Employment. Weaver:Verastem Oncology: Employment, Other: Stockholder; Femto Dx: Equity Ownership; Agios Pharmaceuticals: Employment. Jaeger:Takeda-Millenium: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria; Infinity: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda-Millenium: Membership on an entity's Board of Directors or advisory committees; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; MSD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal